Theme: Embarking Next Generation Delivery Vehicles for Affordable Healthcare
Nanomedicine 2018
Hear Explore and Learn the Latest Research. Present before distinguished global audience. Collaborate, build partnerships and experience Osaka, Japan. Join the Global Nanomedicine Community
ConferenceSeries Ltd invites the contributors across the globe to participate in the premier “18th International Conference on Nanomedicine and Nanotechnology in Health Care” (Nanomedicine 2018) (CPD Accredited), to discuss the theme: "Embarking Next Generation Delivery Vehicles for Affordable Healthcare.” The conference will be held at Osaka, Japan during October 08-09, 2018.
Conference Series Llc organizes a conference series of 1000+ Global Events inclusive of 300+ Conferences, 500+ Upcoming and Previous Symposiums and Workshops in USA, Europe & Asia with support from 1000 more scientific societies and publishes 700+ Open access journals which contains over 30000 eminent personalities, reputed scientists as editorial board members
18th International Conference on Nanomedicine and Nanotechnology in Health Care (Nanomedicine 2018) (CPD Accredited) aims to bring together leading academic scientists, researchers and research scholars to exchange and share their experiences and research results about all aspects of Nanomedicine in Healthcare. It also provides the premier interdisciplinary forum for researchers, practitioners and educators to present and discuss the most recent innovations, trends, and concerns, practical challenges encountered and the solutions adopted in the field of Nanomedicine. The conference program will cover a wide variety of topics relevant to the Nanomedicine, including: Nanomedicine in drug discover and delivery, Nano diagnostics, theranostics, applications of Nanomedicine in healthcare applications and disease treatments.
Why to attend?
18th International Conference and Exhibition on Nanomedicine and Nanotechnology in Healthcare – 2018 (CPD Accredited) which will be the greatest gathering devoted to Nanomedicine and Nanotechnology.
• Professionals giving a chief specialized gathering to detailing and finding out about the most recent new era advancements created over the span of time alongside examining their applications.
• Events incorporate intriguing issues introductions from everywhere throughout the world and expert systems administration with enterprises, driving working gatherings and boards.
• Meet Your Objective Business area with people from and around the world focused on getting some answers concerning Pharmaceutics and Nanomedicine, this is the most obvious opportunity to accomplish the greatest accumulation of individuals from wherever all through the World.
• Conduct appears, scatter information, meet with current, make a sprinkle with another item offering, and get name affirmation at this event. Generally acclaimed speakers, the most recent techniques, methodologies, and the most breakthrough updates in Nanomedicine and Nanotechnology are indications of this meeting.
Target Audience:
Nanomedicine Academia Professors , Medical professionals, Nanomedicine Department heads, Nanomedicine researchers, Nanomedicine CTOs, Nanomedicine product managers, business development managers, Entrepreneurs, Industry analysts, Investors, Students, Media representatives and decision makers from all corners of Nanoscience research area around the globe.
We therefore encourage all colleagues from all over the world to participate and help us to make this an unforgettable important and enjoyable meeting.
We look forward to seeing you in Osaka, Japan !!!
ConferenceSeries Ltd invites all the participants from all over the world to attend “18thInternational Conference on Nanomedicine and Nanotechnology in Health Care” during October 08-09, 2018 at Osaka, Japan. It will include presentations and discussions to help attendees address the current trends and research on the applications of Nanomedicine and nanotechnology in healthcare. The theme of the conference is "Embarking Next Generation Delivery Vehicles for affordable Healthcare"
Nanomedicine is innovating the healthcare industry and impacting our society, but is still in its infancy in clinical performance and applications. The aim of this Nanomedicine 2018conference is to bring together leading academic, clinical and industrial experts to discuss development of innovative cutting-edge Nanomedicine and challenges in Nanomedicine clinical translation.
Track 1: Nanomedicine and Nanobiotechnology
Nano medicine can be defined as medical application of nanotechnology. Nanomedicine ranges from the medical applications of nanomaterial and biological devices, Nano electronic devices & biosensors and possible future applications of molecular nanotechnology. Nanomaterials can be functionalised to interface with biological molecules & structures as the size of nanomaterials is comparable to most biological molecules and structures. Nanomaterials can be useful for both in vivo and in vitro biomedical research and applications and integration of nanomaterials with biology has led to the development of advanced diagnostic devices, physical therapy applications, analytical tools, contrast agents and drug delivery vehicles. Nanomedicine strives for delivering valuable set of research tools & clinically useful devices and its industry sales reached $16 billion in 2015, with an average of $3.8 billion investment in nanotechnology R&D every year and increase of 45% per year global funding for emerging nanotechnology.
Track 2: Nanotechnology in Healthcare
Nano medicine affects almost all the aspects of healthcare. Nano medicine helps to engineer novel and advanced tools for the treatment of various diseases and the improvement of human bio systems using molecular Nanotechnology. Cardiovascular diseases, Neurodegenerative disorders, Cancer, Diabetes, Infectious diseases, HIV/AIDS are the main diseases whose treatment can be benefitted by using Nano medicine.
Track 3: Nano medicine and Nano pharmaceuticals
Nano pharmaceuticals such as liposomes, quantum dots, dendrites, carbon nanotubes and polymeric nanoparticles have brought considerable changes in drug delivery and the medical system. Nano pharmaceuticals offer a great benefit for the patients in comparison with the conventional drugs. There are several advantages of these drugs such as enhanced oral bioavailability, improved dose proportionality, enhanced solubility and dissolution rate, suitability for administration and reduced food effects.
Track 4: Applications of Nanotechnology in Health Care
Nanotechnology has the potential to completely revolutionise all the three key aspects of healthcare sector diagnosis, prevention and Treatment. It can completely change the healthcare sector for the next generation. Nanotechnology will help medical professionals in today's most excruciating medical issues, such as repairing of damaged organs, diagnosis and treatment of cancer cells, removal of obstruction in brain and it can help in better drug delivery system.
Track 5: Nanotechnology for Environment and Nano safety
Nanotechnology in vitality framework science and designing have been looking to grow better than ever sorts of vitality advancements that have the capacity of enhancing life everywhere throughout the world. With a specific end goal to make the following jump forward from the present era of geothermal innovation, researchers and engineers have been creating vitality uses of nanotechnology. BCC Research assesses the aggregate vitality related business sector in Photovoltaic gadgets for Batteries and geothermal nanotechnologies and Nano materials at about $8.8 billion in 2012 and $15 billion. There are 26 colleges and 15 new inquires about is been going on Electrochemistry. The examination includes in atomic responses and Fuel cells.
Track 6: Nanomedicine in Drug Delivery Systems
Nanoparticle technology was recently shown to hold great promise for drug delivery applications in nanomedicine due to its beneficial properties, such as better encapsulation, bioavailability, control release, and lower toxic effect. Despite the great progress in nanomedicine, there remain many limitations for clinical applications on Nano carriers. To overcome these limitations, advanced nanoparticles for drug delivery have been developed to enable the spatially and temporally controlled release of drugs in response to specific stimuli at disease sites. Furthermore, the controlled self-assembly of organic and inorganic materials may enable their use in theranostic applications. This review presents an overview of a recent advanced nanoparticulate system that can be used as a potential drug delivery carrier and focuses on the potential applications of nanoparticles in various biomedical fields for human health care. A novel process for synthesis of polymeric nanoparticles for use in drug delivery applicationsusing the electro spraying technique. The technologyis standardized for synthesis of natural polymer based nanoparticles such as chitosan-gelatin based nanoparticles.
Track 7: Nano in Pharmaceutical Chemistry
A standout amongst the most encouraging nanotechnology fields is Nano pharmaceuticals. Since nanomaterial’s might enter the body through dermal presentation, inward breath, ingestion, or visual contact, they loan themselves to inventive medication conveyance frameworks. Pharmaceutical examination, toxicology thinks about, definition, and assembling of pharmaceutical items require material portrayal to guarantee reliable medication security and viability Nano scale pharmaceutical procedures in medication revelation and advancement outline and improvement of Nano formulations and Nano scale drug conveyance frameworks, administrative viewpoints and approaches identified with Nano pharmaceuticals.
Track 8: Graphene modification and functionalization
Chemical functionalization of graphene enables the material to be processed by solvent assisted techniques, such as layer by layer assembly, spin coating and filtration. Hexagonal boron nitride is electrical insulating, combined with graphene and other 2D materials to make heterostructure devices. The two dimensional graphene sheet structures for field emission of electrons due to the carrier mobility and electron mass. The filed emitter by using multi layered graphene nanostructure, the graphitic structure of pristine graphene and carbon nanotube is the driving force of their interaction .The combination of graphene with carbon nanotubes to produced hybrids increased electrical conductivity, mechanical properties and high surface area.
Track 9: Advanced Characterization of Nanomaterials
Advances in nanotechnology have opened up a new era of diagnosis, prevention and treatment of diseases and traumatic injuries. Nanomaterial’s, including those with potential for clinical applications possess novel physicochemical properties that have an impact on their physiological interactions, from the molecular level to the systemic level. There is a lack of standardized methodologies or regulatory protocols for detection or characterization of nanomaterial. This review summarizes the techniques that are commonly used to study the size, shape, surface properties, composition, purity and stability of nanomaterial’s, along with their advantages and disadvantages. At present there are no FDA guidelines that have been developed specifically for nanomaterial based formulations for diagnostic or therapeutic use. There is an urgent need for standardized protocols and procedures for the characterization of nanoparticles, especially those that are intended for use as theranostics.
Track 10: Nano Composites and Multiscale Nano materials
In the large field of nanotechnology, polymer matrix based Nano composites have become a prominent area of current research and development. Exfoliated clay based Nano composites have dominated the polymer literature but there are a large number of other significant areas of current and emerging interest. Polymer nanotube Nano composite conducts electricity.
Track 11: Nano materials and Nanotechnology
Nanotechnology is the handling of matter on an atomic, molecular, and supramolecular scale. The interesting aspect about nanotechnology is that the properties of many materials alter when the size scale of their dimensions approaches nanometres. Materials scientists and engineers work to understand those property changes and utilize them in the processing and manufacture of materials at the Nano scale level. The field of materials science covers the discovery, characterization, properties, and use of Nano scale materials.
Track 12: Biomaterials and Medical Devices
Biomaterials from healthcare viewpoint can be defined as materials those possess some novel properties that make them appropriate to come in immediate association with the living tissue without eliciting any adverse immune rejection reactions. Biomaterials are in the service of mankind through ancient times but subsequent evolution has made them more versatile and has increased their usage.
Track 13: Nanotechnologies and commercial viability
Nano science and Molecular Nanotechnology is the new outskirts of science and innovation in Europe and around the globe, working at the size of individual particles. Top researchers and in addition policymakers overall acclaim the advantages it would convey to the whole society and economy: a large portion of them demand the key part research would play in the quality creation procedure to create exploitable arrangement of innovations by the European business prompting a decision of remarkable applications, items, markets and productive income sources.
Related Conferences:
- Nano science and Molecular Nanotechnology Conferences,
- 12th International conference and expo on Nano Science and Molecular Nanotechnology October 20-22, 2018 Rome, Italy.
- 12th Nanotechnology Products Expo November 24-25, 2018, Melbourne, Australia
- 2nd World Congress and Expo and Expo on Medical Devices, December 01-03, 2018, Baltimore, USA
- Conference on Nanoscience and Nanotechnology (ICONN), Canberra, Australia
- The Fundamental Science of Nanotechnology, Oxford, United Kingdom,
- 4th Nanotechnology-2018, Dubai, UAE
- High Performance and Optimum Design of Structures and Materials 2018 Siena, Italy
- 6th Advanced Materials Research conference (ICAMR 2018) Torino, Italy
Related Societies:
- American Nano Society
- European Biotechnology Thematic Network Association
- Society for Biomaterials
- Nano Canadian Society
- American Academy of NanoMedicine
- American Association for the Advancement of Science
- Nanometer-Scale Science and Technology Division of the American Vaccum Society
- NanoScience and Technology Institute
- ASME NanoTechnology Institute
- Foresight Nanotech Institute
- International Association of NanoTechnology
- The Institute of NanoTechnology
- Microscopy Society of America
- Nano Business Alliance
- European NanoTechnology Gateway
- Scottish Center for NanoTechnology in Construction Materials
- Royal Society-NanoTechnology and NanoScience
- Czech NanoTechnology Industries Association
- Erwin Schrodinger Society for NanoSciences
- Innovationsallianz Carbon NanoTubes
- NanoTechnologies for Tommorow's Society
- American Association for the Advancement of Science
18th International conference on Nanomedicine and Nanotechnology in Healthcare | October 08-09, 2018 Osaka, Japan.
Nanomedicine 2018 is an emerging field of engineering and life sciences that promises to revolutionize medicine and medical technology. There are numerous applications of nanomedicine and nanodelivery using Nanotechnology in medicinal diagnostics. These include improved imagining of the human (or any) body and detecting tumors that are only a few cells in size.
The idea that pharmaceutical agents should be delivered specifically to diseased cells holds the promise of a variety of benefits. The promise of individualized medicine is that it is efficient. Targeted drug-delivery allows doctors and patients to benefit from small dosages at just the right place and thus from fewer side effects.
Nanomedicine has therapeutic uses as well. Nanotechnology is capable of delivering medication to the exact location where they are needed, hence lesser side effects. It can also be used to destroy harmful organisms or cancer cells by interrupting their division process. Nanoprobes can be made to generate radiation that could kill bacteria, viruses and cancer cells. Nanotechnology also theoretically allows the mimicking of natural biological processes, e.g. repair of damaged tissues or acting as artificial red blood cells to transport oxygen.
The global market for healthcare nanotechnology is expected to reach USD 196.02 billion by 2020 growing at a CAGR of 12.1%, according to a new study by Grand View Research, Inc. Increasing susceptibility of patients towards chronic diseases such as cardiovascular, neurological, oncology and respiratory diseases coupled with increasing R&D spending opening new application avenues is expected to drive market growth over the next six years. Other drivers of this market include increasing government and private sector R&D aid and new players entering the market to bridge the gap between supply and demand.
Importance & Scope of Nano Medicine:
Researchers are developing a nanoparticle that can be taken orally and pass through the lining of the intestines into the bloodstream. This should allow drugs that must now be delivered with a shot to be taken in pill form. The researchers have demonstrated the technique with lab mice so far.
Researchers are also developing a nanoparticle to defeat viruses. The nanoparticle does not actually destroy viruses molecules, but delivers an enzyme that prevents the reproduction of viruses molecules in the patients bloodstream. The effectiveness of the technique has been demonstrated in lab tests.
About venue:
Japan is the second-largest individual pharmaceutical market in the world. It accounts for less than 10% of the total global pharmaceutical market. The country’s pharma market is expected to grow annually by ~2.2%. Shionogi & Co's new pharmaceutical research facility is located in Toyonaka City in Osaka, Japan. Osaka is a large port city and commercial centre on the Japanese island of Honshu. Osaka is famous for modern architecture, nightlife and hearty street food. The 16th-century shogunate Osaka Castle, which has undergone several restorations, is its main historical landmark. It's surrounded by a moat and park with plum, peach and cherry-blossom trees. Sumiyoshi-taisha is among Japan’s oldest Shinto shrines.
Osaka is the second largest metropolitan area in Japan and serves a major economic hub. Historically a merchant city, Osaka has also been known as the “Nation’s Kitchen”. With a population of 2.5 million, Osaka is Japan’s third largest and second most important city. It has been the economic powerhouse of the Kansai region for many centuries. The city’s west side has the main port as well as a tourist destination with attractions such as Kyocera Dome, Universal Studios Japan, Osaka aquarium, Minami, Osaka castle, Umeda sky building and the Tempozan Harbour Village. Osaka is known for its food, both in Japan and abroad. Author Michael Booth and food critic François Simon of Le Figaro have both suggested that Osaka is the food capital of the world. Osaka’s culinary prevalence is the result of a location that has provided access to high quality ingredients, a high population of merchants, and close proximity to the ocean and waterway trade. In recent years, Osaka has started to garner more attention from foreigners with the increased popularity of cooking and dining in popular culture. The National Museum of Art (NMAO) is a subterranean Japanese and international art museum, housing mainly collections from the post-war era and regularly welcoming temporary exhibitions. Osaka Science Museum is in a five storied building next to the National Museum of Art, with a planetarium and an OMNIMAX theatre. The Museum of Oriental Ceramics holds more than 2,000 pieces of ceramics, from China, Korea, Japan and Vietnam, featuring displays of some of their Korean celadon under natural light.
Why to Attend??? :
18th International Conference and Exhibition on Nanomedicine and Nanotechnology in Heathcare - 2018 which will be the greatest gathering devoted to Nanomedicine and Nanotechnology.
• Professionals giving a chief specialized gathering to detailing and finding out about the most recent new era advancements created over the span of time alongside examining their applications.
• Events incorporate intriguing issues introductions from everywhere throughout the world and expert systems administration with enterprises, driving working gatherings and boards.
• Meet Your Objective Business area with people from and around the world focused on getting some answers concerning Pharmaceutics and Nanotechnology , this is the most obvious opportunity to accomplish the greatest accumulation of individuals from wherever all through the World.
• Conduct appears, scatter information, meet with current, make a sprinkle with another item offering, and get name affirmation at this event. Generally acclaimed speakers, the most recent techniques, methodologies, and the most breakthrough updates in Pharmaceutics and Engineering are indications of this meeting.
Nanomedicine Market in Japan:
Nanomedicine is a promising sub-segment in medicine that took off in the 1980s with the first generation of developed nanopharmaceuticals. With the use of nanotechnology, drugs can be delivered in ways not experienced so far.U.S. is a strong actor in this field with many patents having commercialized several nanopharmaceuticals.
The global nanomedicine market was valued at US$50.1 billion in 2011 and is projected to grow to US$96.9 billion in 2016. The share of nanomedicine to the total global pharmaceutical market is estimated at 5.3 percent in 2011 indicating its niche character presently.
In Japan, for various reasons, the nanomedicine market size in terms of the total market is much smaller. A rough estimate shows that the share is between 1 to 2 percent corresponding to approximately US$1 to 2 billion. A limited number of approved Japanese nanodrugs together with a long time until approved foreign products entered the Japanese market have seemingly slowed the market expansion.
Japan = World's 2nd largest pharmaceutical market!
- Japan is the 2nd largest pharmaceutical market in the world, marking JPY 10 trillion (USD 84.4 billion) in ethical drug in 2016.
- Nikkei Stock Average has been continuously increasing since mid October, 2016.
- The government is to ease the regulations to develop new medicines for intractable diseases by shortening 5 years of drug development time.
- Japan hugely depends on imported pharmaceutical products. The annual import value in Japan was JPY 1.94 trillion (USD 16 billion) in 2015 compared to the export value of JPY 0.32 trillion (USD 2.7 billion).
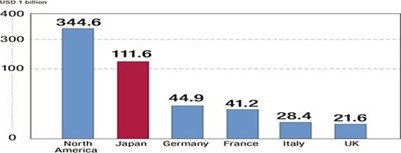
Japan’s pharmaceutical industry is the world’s second largest market, after U.S., valued at US$112.1 billion in 2012 or 11.6 percent of the world market. Historically, the market has been protected from foreign competition. These days, however, deregulation has prompted investment from abroad and increased the presence of foreign companies. The pharmaceutical industry is one of the few industrial sectors in which Japan has a trade deficit. Japan imports more than two times what it exports. The rapid aging of the population and the weak global competitiveness of domestic companies are contributing factors to the trade deficit.
Japan Nanomedicine Market Size:
There is no market information available on the size of Japan’s nanomedicine market published by any of the large Japanese market research companies. Table 2 below tries to estimate the market size. The global nanomedicine market was estimated to be about 5 percent of the global pharmaceutical market in 2010 and 2011. In case of Japan, this ratio is much lower compared to the global nanomedicine market. A rough estimate indicates that the market size was approximately 1-2 percent of the Japanese pharmaceutical market in 2011-2012, or roughly between US$1 billion – US$2 billion. The drug lag of imported nanopharmaceuticals is one cause of this. Nanomedicines have not been defined in Japan and are regulated within the general framework of the Pharmaceutical Affairs Law (PAL) on a product-by-product basis .
Approved Nanopharmaceutical Products by Application:
As there is no specific definition for drug and device (nanocarrier) combinations, they are regulated as drugs or medical devices according to their main function or purpose.
Pharmaceuticals are classified as nanomedicine by their sizes, i.e. materials in the submicron range.Information on marketed nanopharmaceuticals in Japan comes from various sources including “Current Initiatives in Japan for Nanomedicines”, Kumiko Sakai-Kato, Toru Kawanishi, 2011, National Institute of Health Sciences (NIHS) and Ministry of Health, Labour and Welfare (MHLW) .
- Unit: USD Billion Source: Japan Pharmaceutical Manufacturers Association “Data Book 2016”
|
Trade name |
Technology |
Compound |
Company |
|
Ropion |
Lipid emulsion |
Flurbiprofen |
Kaken (JPN) |
|
Visudyne |
Liposome |
Verteporfin |
Novartis |
|
Zevalin |
Antibody Conjugates |
Zevalin |
Bayer |
|
Somavert |
PEGylated protein |
Pegvisomant |
Pfizer |
|
Emend |
Nanocrystal |
Aprepitant |
Merck |
|
Abraxane |
Polymeric Nanoparticles |
Paclitaxel |
Abraxis |
Government Pushing for Change:
Order to reduce the time span from discovery and innovation to commercialization, the importance to establish open user facility networks to promote the integration of dissimilar fields and academic-industry collaboration is emphasized.
It is apparent that the government is aiming at more concrete and speedy results for R&D. Issue-driven innovation based on “exit-oriented” R&D is targeted to impact the competitive power of related industries.
A report by the Japan Science & Technology Agency titled “Japan’s New Science and Innovation Policy – Beyond the Boundaries for Innovation”, published in 2016 (50) lists up the time span for selected target applications of nanomedicine, such as:
- Molecular imaging (2015-2020)
- Integrated system of drug delivery, diagnosis and treatment (2015-2020)
- Implant devices for diagnosis and treatment (2020-2030)
- Nano-cell surgery (2020-2030)
- 3D-imaging in cells (2020-2030)
These are quite ambitious targets showing the directions where R&D will be focused.
In addition to the latest basic plan, there are other signals that the government is increasingly prioritizing innovative medicine. For instance, The Ministry of Health, Labour and Welfare will jointly with the European Union (EU) promote the development of nano-based block copolymer micelles. Together with European Medicines Agency (EMA) the ministry has released a reflection paper (February 2013) emphasizing that such micelles are able to preferentially accumulate in solid tumors (51).
Nanomedicine – Research and Development in Japan:
- University of Tokyo
- Hokkaido University
- Osaka Prefecture University
- Osaka University
- Tohoku University
- National Institute for Materials Science
The Japanese Nanomedicine Industry:
Nanomedicine start-ups and small-medium enterprises have driven the innovation process, not only in US and Europe but also in Japan. The commercialization of nanopharmaceuticals have basically followed three types of business models , such as:
- Development of a nanotechnology platform used to add value to second-party products
- Development and manufacturing of high-value materials for the pharmaceutical industry
- Development of nanotechnology-improved pharmaceuticals or medical devices
The majority of start-ups has adopted the third business model utilizing nanotechnology to develop own proprietary product pipelines. Often such companies introduce new or standard drugs that are delivered with a drug delivery system. Then they try to team up with pharmaceutical companies that take the products through the clinical trials.
- NanoCarrier Co., Ltd.
- LTT Bio-Pharma Co., Ltd.
- Mebiopharm Co., Ltd.
- Nippon Kayaku Co., Ltd
- Kowa Company Ltd.
- Mitsubishi Tanabe Pharma Corporation
- Taisho Pharmaceutical Co., Ltd.
- Astellas Pharma Inc
- Kaken Pharmaceutical Co., Ltd.
Drug Delivery Devices market in Japan:
Japan has one of the world’s most advanced healthcare facilities. The healthcare administration in Japan is also becoming a model for many developing nations.
The report details several factors driving the market, some of which are listed below.
Drivers
· Increased prevalence of chronic diseases
· Advances in drug delivery system
· Increase in individual therapy
· Increased understanding about drug metabolism among the population
· Increasing demand for generic drugs
· Requirement of controlled drug release
Japan’s drug delivery market has been segmented into oral drug delivery systems, nasal drug delivery system, pulmonary drug delivery system, injectable drug delivery, ocular drug delivery, topical drug delivery, transmucosal drug delivery and implantable drug delivery. The segmentation on oral drug delivery system is sub-divided into solid oral drugs (includes tablets, capsules, powders, and pills), liquid oral drugs (includes syrups and solutions), semi-solid oral drugs (includes gels, emulsions, suspensions, and elixirs). The segmentation on nasal drug delivery systems has been subdivided into nasal drops, nasal sprays, nasal powders, nasal gels. The segmentation on pulmonary drug delivery systems includes metered dose inhalers, dry powder inhalers, and nebulizers. The segmentation on injectable drug delivery has been further segmented into general injectable, self -injection device, needle-free injectors, auto-injectors, and pen injectors.
The global drug delivery system market size was valued at USD 379.2 Billion in 2015. Growing efforts towards formulating dosage forms that would help in maximizing the bioavailability of the drug at the target site while increasing the patient convenience are driving the global market growth. The introduction of the novel drug delivery systems such as subdermal implants that are available in different forms such as rings and patches are expected to aid in the industry growth.
The evolution of the drug delivery systems is directly attributed to changing preferences by patients as well as healthcare professionals. A few of the many advancements include-
• Introduction of Biopharmaceutical classification systems (BCS) class II drug in oral dosage forms
• Introduction of auto-injectors
• Availability of advanced implants in customized shapes and sizes
• Introduction of advanced pulmonary devices such as Smartcard and Adaptive Aerosol Delivery
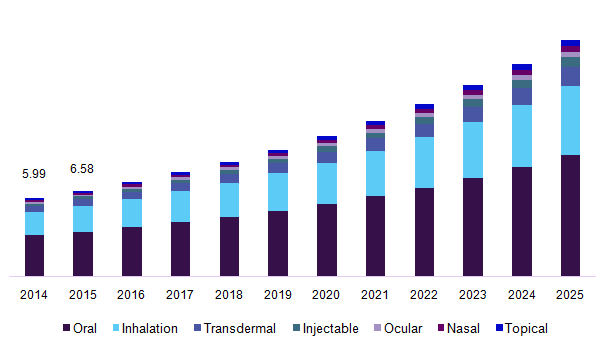
Japan drug delivery systems market, by route of administration, 2014 - 2025 (USD Billion)
- Pharmaceutical Regulations in Japan:
- Manufacturing, importation, and sales of drugs and medical devices are regulated by the Pharmaceutical Affairs Law (PAL) of Japan.
- All manufacturing and marketing applications in Japan for drugs and devices are reviewed by the Pharmaceutical and Medical Devices Agency (PMDA) . All applications are thoroughly reviewed before PMDA submits an approval recommendation to the Ministry of Health, Labour and Welfare (MHLW).
- Under PAL, when importing to Japan and selling pharmaceutical products manufactured in other countries, a license for marketing authorization is required. The Marketing Authorization Holder (MAH) will be the owner of the license for marketing authorization.
- The MAH must be based in Japan and can be the foreign company’s Japan office, the foreign company’s distributor, or an independent third party acting as the Designated Marketing Authorization Holder (DMAH).
- To import and market a new drug in Japan, an approval (marketing approval) will be necessary. And the approval must be held by the Marketing Authorization Holder.
- A foreign manufacturer intending to manufacture drugs in foreign countries and export them to Japan, is required to be accredited by MHLW as an “Accredited Foreign Manufacturer” (84). And it is necessary to obtain accreditation for each foreign factory location at which pharmaceuticals for export are manufactured.
- The appointed MAH will be responsible for the labelling and advertising of the pharmaceuticals in Japan. As stipulated in PAL, the manufacturer’s/seller’s address, name of product, production indication, name of ingredients, expiration, etc., must be printed on the container of drugs.
- Overall research in various disciplines:
The North American Nano medicine market held the majority of global market share in 2012 because of the rapidly growing nanomedicine market in the Asia-Pacific, Latin American and African region, presence of large number of patented nanomedicine products and favorable regulatory framework in the region. In addition, the presence of sophisticated healthcare infrastructure supports development of advanced products such as nano probes, nanorobots, monoclonal antibody based immunoassays and nanoparticle based imaging agents for early detection of diseases.
However, the Asia-Pacific region is expected to grow at a faster CAGR owing to presence of high unmet healthcare needs, research collaborations and increase in nanomedicine research funding in emerging economies such as China, India and other economies in the region. China is expected to surpass the United States in terms of nanotechnology funding in the near future, which indicates the growth offered by this region.
Major Nano Medicine Associations around the Globe:
- American Nano Society
- European Biotechnology Thematic Network Association
- Society for Biomaterials
- Nano Canadian Society
- American Academy of NanoMedicine
- American Association for the Advancement of Science
- Nanometer-Scale Science and Technology Division of the American Vaccum Society
- NanoScience and Technology Institute
- ASME NanoTechnology Institute
- Foresight Nanotech Institute
- International Association of NanoTechnology
- The Institute of NanoTechnology
- Microscopy Society of America
- Nano Business Alliance
- European NanoTechnology Gateway
- Scottish Center for NanoTechnology in Construction Materials
- Royal Society-NanoTechnology and NanoScience
- Czech NanoTechnology Industries Association
- Erwin Schrodinger Society for NanoSciences
- Innovationsallianz Carbon NanoTubes
- NanoTechnologies for Tommorow's Society
- American Association for the Advancement of Science
Companies involved in Nano Technology:
USA
- Oncolytics Biotech
- Bristol-Myers Squibb
- GlaxoSmithKline
- Bend Research
- Pfizer
- BioDelivery Sciences
- GE Healthcare
- Mallinckrodt plc
- Nanosphere Inc., USA
- Pfizer Inc., USA
- Merck & Co Inc., USA
- Celgene Corporation, USA
- CombiMatrix Corporation, USA
- Abbott Laboratories
- Many Major companies in the Nano Delivery market.
Global
Conference Highlights
- Nanotechnologies and commercial viability
- Biomaterials and Medical Devices
- Nanomaterials and Nanotechnology
- Nano Composites and Multiscale Nano materials
- Advanced Characterization of Nanomaterials
- Graphene modification and functionalization
- Nano in Pharmaceutical Chemistry
- Nanomedicine in Drug Delivery Systems
- Nanotechnology for Environment and Nano safety
- Applications of Nanotechnology in Health Care
- Nanomedicine and Nano pharmaceuticals
- Nanotechnology in Healthcare
- Nanomedicine and Nanobiotechnology
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | October 08-09, 2018 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Nanomedicine & Nanotechnology
- Journal of Nanomedicine & Biotherapeutic Discovery
- Journal of Nanomaterials & Molecular Nanotechnology
Abstracts will be provided with Digital Object Identifier by




















